Chem. Commun., 2013, 49,7201-7203
DOI: 10.1039/C3CC44312D, Communication
DOI: 10.1039/C3CC44312D, Communication
Cesar A. Urbina-Blanco, Maciej Skibinski, David O'Hagan, Steven P. Nolan
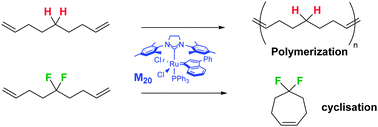
The gem-difluoromethylene (CF2) group significantly accelerates ring-closing metathesis of 1,8-nonadienes relative to the methylene (CH2) group demonstrating similar rate accelerations to that observed for the classic Thorpe-Ingold substituents, diester malonates and ketals.
The content of this RSS Feed (c) The Royal Society of Chemistry
The gem-difluoromethylene (CF2) group significantly accelerates ring-closing metathesis of 1,8-nonadienes relative to the methylene (CH2) group demonstrating similar rate accelerations to that observed for the classic Thorpe-Ingold substituents, diester malonates and ketals.
The content of this RSS Feed (c) The Royal Society of Chemistry